Recent scientific advances revealed that certain glycan (complex carbohydrate) patterns on cell surfaces are drivers of immune suppression in a high percentage of cancer patients and can also impact disease progression and therapeutic response in many autoimmune and inflammatory diseases. Scientific nuances inherent to glycobiology limit conventional approaches to drug discovery in this emerging field. Palleon has created a proprietary drug discovery platform which overcomes technical barriers and uniquely enables therapeutic targeting of pathologic cell surface glycans as well as the immune receptors that detect them.
Our Approach
EAGLE Platform
Sialoglycans are a family of glycans found on cell surfaces that normally prevent the immune system from attacking healthy cells, but they can be co-opted as camouflage by pathogenic cells driving autoimmunity and cancer. Sialoglycan biology offers great potential for developing new therapies for these diseases but is difficult to target with conventional drug development strategies. Whereas protein-protein interactions typically rely on one-to-one binding relationships that can be targeted and disrupted by a single drug, sialoglycans and their receptors—like the clingy surface of Velcro—work through many touchpoints and therefore require a new approach.
Palleon’s EAGLE (Enzyme-Antibody GLycan Editing) Platform enables the targeting of all sialoglycan touchpoints on cell surfaces simultaneously by removing their terminal sialic acid molecules, making pathogenic cells vulnerable to clearance by the immune system. The EAGLE platform can be applied to both autoimmunity and cancer.
A New Frontier in Biology
Palleon Scientific Co-Founder and Nobel laureate Carolyn Bertozzi’s discoveries in the field of glycobiology enabled the development of Palleon’s glycan editing therapeutic platform.

The Opportunity in Autoimmunity
B cells – the immune cells that make antibodies – are critical for immune defense against pathogens, but they can become dysfunctional in autoimmunity when they target the body’s own tissues. Depleting these pathogenic B cells with targeted antibodies such as rituximab (anti-CD20) is an established treatment for many autoimmune diseases. However, some patients have inadequate clinical response to these drugs, and many who do respond still require large doses of steroids.
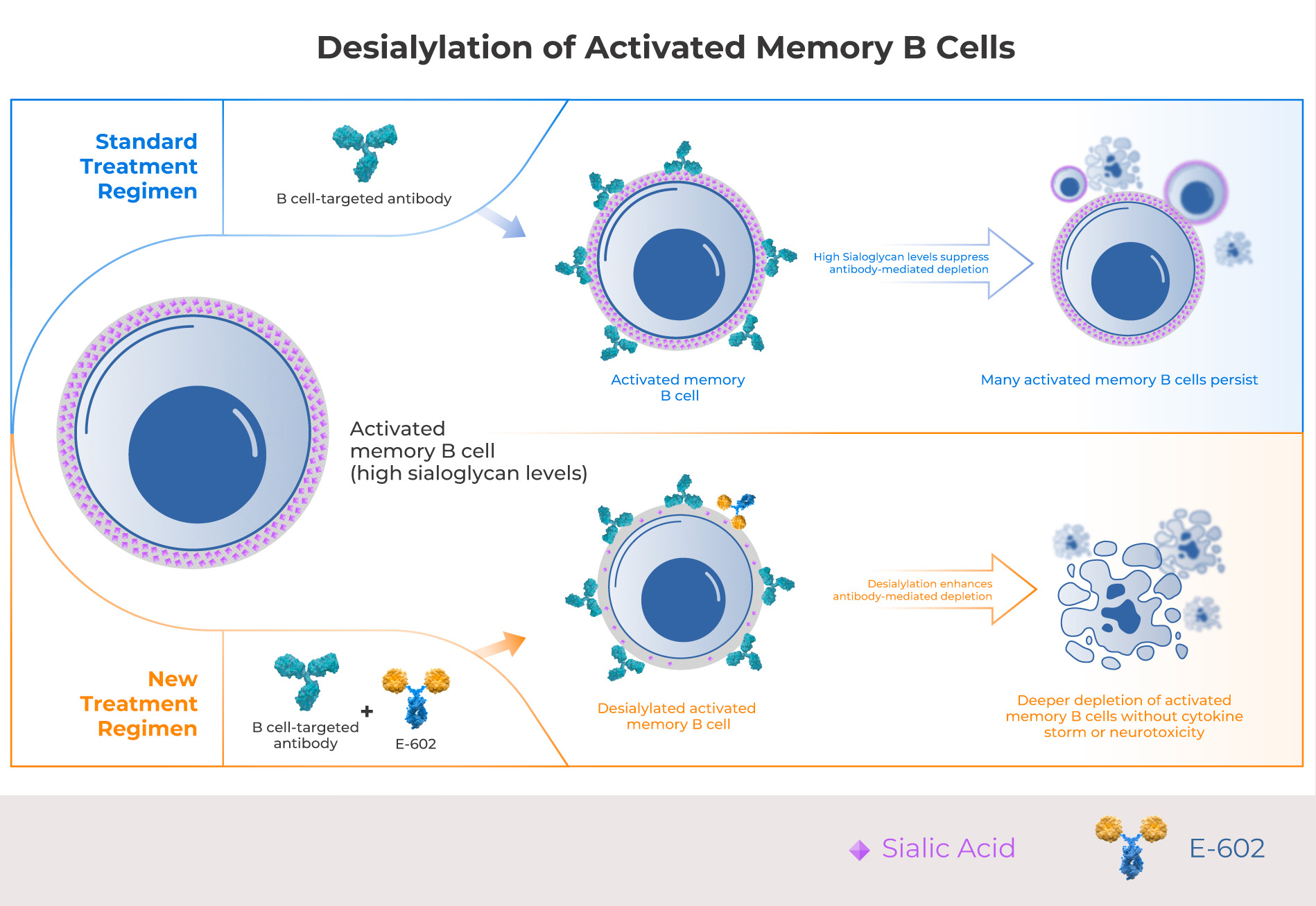
Activated memory B cells are the subpopulation of B cells most responsible for autoimmune pathologies. While all B cell subsets have sialoglycans, memory B cells have the highest levels of sialylation, making them more resistant to antibody-mediated depletion. Palleon’s lead candidate E-602 enzymatically degrades sialoglycans on activated memory B cells, enabling B cell-targeted antibodies to deplete memory B cells more efficiently.
Creating safe and deep B cell depletion for the outpatient setting
Recent success with CD19 CAR T therapy and T cell engagers has strengthened the hypothesis that deeper B cell depletion can achieve meaningful clinical results in autoimmunity, however, these approaches have severe toxicity risks including cytokine storm and neurotoxicity. E602 has a proven safety profile in humans and is expected to enhance depletion of autoreactive memory B cells without the safety risks associated with emerging T cell therapies, making it suitable for the community outpatient setting.
Targeted Sialidase Molecules in Cancer
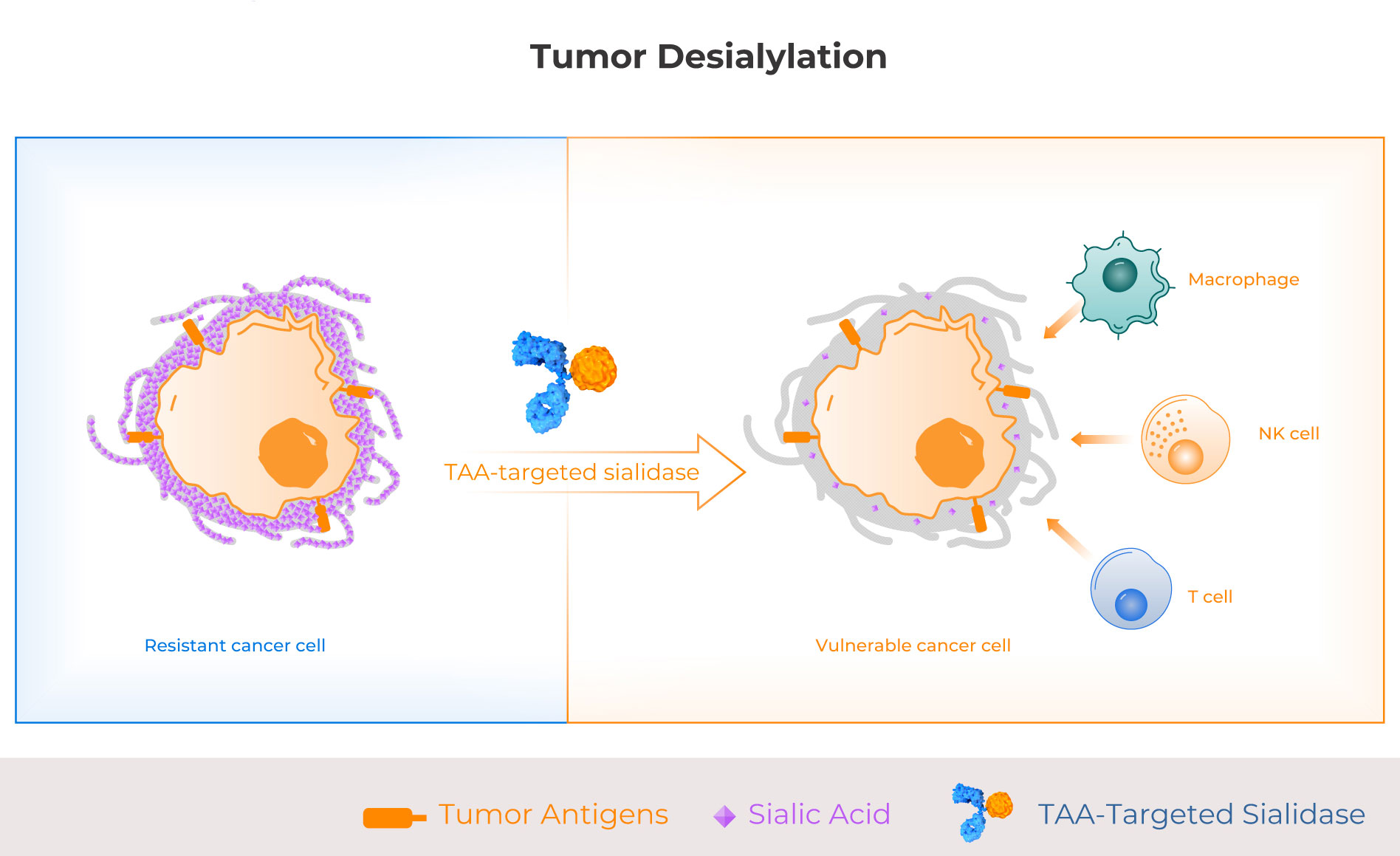
The majority of solid tumors have hypersialylation, especially lung cancer, ovarian cancer, and colon cancer, and preclinical and human translational evidence demonstrates that hypersialylation is a major driver of immune suppression and resistance to therapy. Palleon is developing targeted sialidase molecules comprising a human sialidase enzyme and a tumor antigen targeting arm. These first-in-class glycan editors enzymatically degrade immunosuppressive sialoglycans on hypersialylated tumors, making them more vulnerable to anti-tumor immunity.
HYDRA Platform
Palleon has developed the powerful HYDRA platform to support our portfolio of therapeutic candidates. HYDRA is an immunohistochemistry-based translational research technology that measures the density of hypersialylation on tissue samples. It provides a clinical biomarker and could potentially be used in the future as a companion diagnostic.