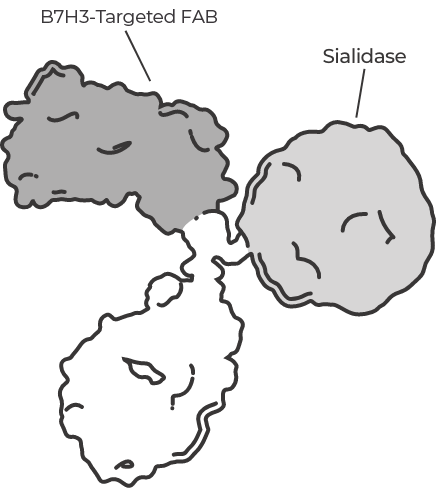
E-688 is a novel glycan-editing therapeutic from Palleon’s EAGLE platform. The EAGLE (Enzyme-Antibody Glycan-Editing) platform enables creation of novel biological therapeutics by genetic fusion of human sialidase with human monoclonal antibodies. E-688 is designed to enhance the desialylation of tumor cells that express B7H3.
In June 2022 Palleon entered into a strategic collaboration with Henlius to co-develop targeted sialidase molecules. Under the terms of the agreement, Palleon will perform research and the parties will then share preclinical and global clinical development responsibilities and costs for E-688. Henlius has an exclusive license to E-688 in Greater China, while Palleon retains all other global rights.