New research suggests that the upregulation of sialoglycans – complex sugar chains that terminate with a sialic acid and coat cell surfaces – suppresses the activation of the immune system in more than 50% of cancer patients. Both tumor cells and immune cells can become hypersialylated, contributing to immune evasion in cancer. Dysregulated glycans are also linked to several inflammatory disorders including rheumatoid arthritis, idiopathic pulmonary fibrosis, and autoimmune vasculitis. Palleon’s scientific co-founder Dr. Carolyn Bertozzi was awarded the 2022 Nobel Prize in Chemistry for the invention of bioorthogonal chemistry, a discipline which made possible the foundational discoveries enabling Palleon’s glycan editing therapeutic platform.
Sialoglycan-Mediated Immune Suppression in Cancer


Our Approach
The Challenge in Cancer
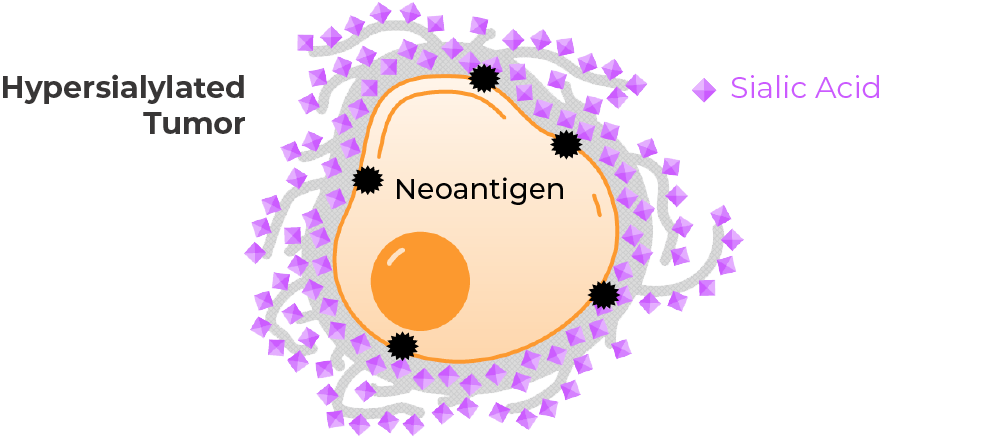
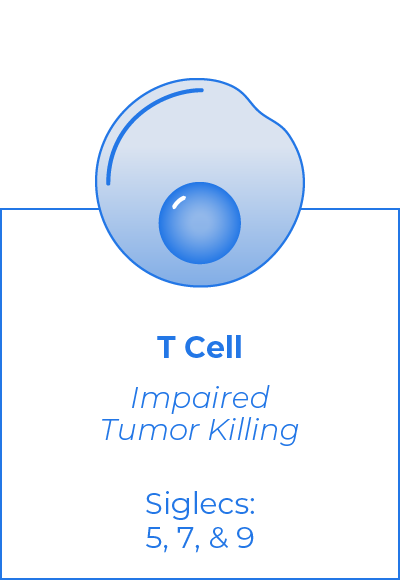
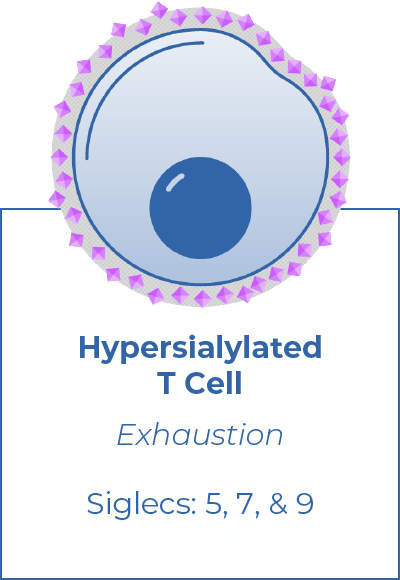
Hypersialylation of tumor and immune cell surfaces is a major cause of immune evasion in cancer. Sialoglycan-mediated immune suppression involves over a dozen inhibitory checkpoint receptors, each of which can bind to many different sialoglycans. Conventional drug development – which targets one-to-one binding relationships – is not applicable to this axis, where there is too much redundancy to effectively target a single receptor or ligand.
EAGLE Platform
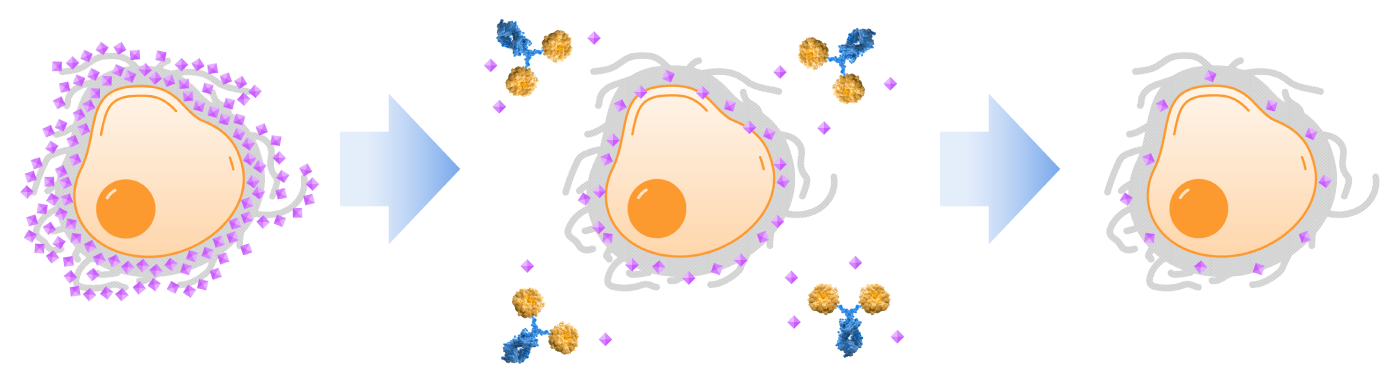
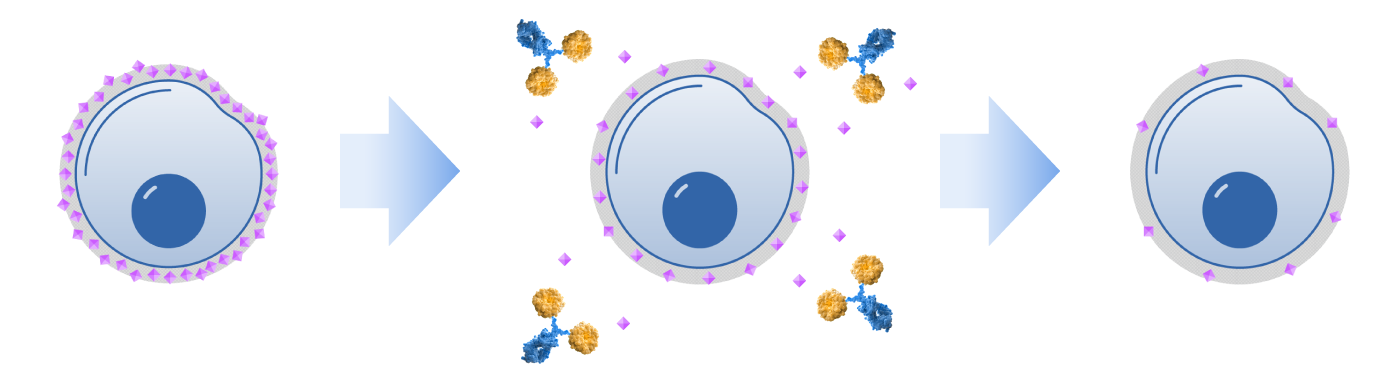
Palleon researchers discovered the Achilles heel of sialoglycan-mediated immune evasion – many glycan-sensing receptors that block the immune system depend on the presence of sialic acid. The EAGLE platform enables the development of therapeutics that strip sialic acid — the critical common dependency of this axis — from both cancer cells and immune cells. Desialylation enables immune cells to launch a comprehensive antitumor response with immune memory.
The EAGLE platform is based on engineered human sialidase enzymes. Palleon’s lead candidate, E-602 (Bi-Sialidase) is a first-in-class, glycan editor which restores antitumor immunity by enzymatically degrading immunosuppressive sialoglycans on hypersialylated tumors and immune cells. It is currently being evaluated in patients with advanced cancer in a Phase 1/2 trial. Phase 1 results from this trial demonstrated proof of mechanism, including dose dependent desialylation and dose dependent immune system activation. Additionally, E-602 was found to be well tolerated across the entire dose range evaluated with no dose limiting toxicities. Palleon is also developing targeted sialidase molecules which are comprised of a human sialidase enzyme and a targeting arm.

HYDRA Platform
Palleon has developed the powerful HYDRA platform to support our portfolio of cancer therapeutic candidates. HYDRA is an immunohistochemistry-based translational research technology which quantifies immunosuppressive sialoglycan density in cancer patient samples for clinical development prioritization and evaluation of pharmacodynamic activity. By identifying tumor types that are most frequently hypersialylated, HYDRA allows Palleon to de-risk drug development.
Autoimmune Diseases
Changes in cell surface glycosylation can also contribute to immune dysfunction in inflammatory and autoimmune diseases. Research has shown a correlation between low levels of sialylation and autoimmunity. Targeting sialoglycans and/or their receptors could have therapeutic potential in suppressing the immune system in these diseases. In addition, targeting sialoglycan biology in combination with other agents could address refractory autoimmunity in some patients. Palleon is developing multiple strategies, including glycan-editing and Siglec-targeting programs to address this therapeutic area.
